The Silica
Introduction
“silica” refers to silicon dioxide compounds, SiO 2, in their various forms including crystalline silicas; glassy silicas and amorphous silicas. Silicon dioxide, SiO2, is the most common binary oxygen and silicon compound, and is even composed of the two most abundant elements in the Earth’s crust. Silica and its compounds make up about 60% by weight of the entire earth’s crust.
Silica deposits are universally found and come from various geological eras. Most silica deposits that are mined for “silica sands” consist of free quartz, quartzites, and sedimentary deposits such as sandstones.
Quartz is a mineral of a hard, inert and insoluble nature. It fully supports various weathering processes and is found from trace to large quantities in various sedimentary rocks. It is the major component of soils, ranging from 90 to 95% of the sandy and silt fractions of a soil. The sand is predominantly composed of quartz.
Commercially, silica is the source of the silicon element and is widely used as a constituent of building materials. Silica also has numerous specialized applications such as piezoelectric crystals. In its amorphous form it is used as a desiccant, adsorbent, filler and catalyst component. In its glassy form it is widely used in the glass industry and as optical components. Silica is a basic material in the glass, ceramic and refractory industry, and is an important raw material in the production of soluble silicates, silicon and its silicon carbide derivatives and silicones.
Due to its abundance in the earth’s crust, silica is widely used as a constituent of numerous materials. This way, workers can be exposed to crystalline silica in a wide variety of industries and occupations. Table 1 provides examples of specific industries, operations and activities where occupational exposure to free crystalline silica may occur. Crystalline silica refers to a mineral group in which silica assumes a regularly repeating structure, ie a crystalline structure.
Eight different SiO2 structural arrangements (polymorphs) occur in nature, but seven of these are most important in the conditions of the earth’s crust: a-quartz, cristobalite, tridymite, moganite, keatite, coesite, and stishovite.
The three most important forms of crystalline silica from an occupational health standpoint are quartz, tridymite and cristobalite. These three forms of silica are also called free silica or uncombined silica to distinguish them from other silicates.
Table 1 Branches of activity where occupational exposure to crystalline free silica may occur.
| Industry / Activity | Specific Operation / Task | Source of material |
| Agriculture | Plowing, harvesting, machine use | Solo |
| Mining and operations related to the processing of
ore |
Most occupations (underground, shallow,
mill) and mines (metal, not metal, coal) |
Ores and associated rocks |
| Mining / extraction and operations related to the
ore beneficiation |
Process of grinding stone, sand and gravel, cutting Stonework, abrasive blasting, slate work, The calcination of diatomite | Sandstone, granite, stone, sand, boulder, slate,
diatomaceous earth, stone. |
| Construction | Abrasives for blasting structure, buildings.
Highway and tunnel tunnels.Excavation and handling of
earth.Masonry, concrete work, demolition. |
Sand and concrete.RochaSolo and rockConcrete, mortar and
trailer. |
| Glass including fiberglass | Glass including fiberglass | Sand, ground quartzRefractory material. |
| Cement | Raw Material Processing | Clay, sand, limestone, diatomaceous earth. |
| Abrasives | Silicon Carbide ProductionProduct Manufacturing
Abrasives |
Sand, tripoli and sandstone |
| Ceramics including bricks, tile, sanitary porcelain,
porcelain, pottery, refractory, glazed enamels. |
Mixtures, molding, Glazed or enamelled coatings,
finishing. |
Clay, stone, sand “Shale” Quartzite, diatom earths. |
| Iron and steel fabrication | Manufacture (handling) of refractories and repairs to
ovens |
Refractory Material |
| Silicon and ferro-silicon | Handling of raw materials | Sand |
| Foundries (ferrous and non-ferrous) | Part casting, shocks to remove part from
mold.Cleaning of the piece that has sand adhered to the surface. Use of abrasive. Smoothing / flattening operations. Furnace repair and repair. |
SandSandRefractory Material. |
| Metal products, including structural metal, machinery,
transport equipment. |
Blasting abrasive | Sand |
| Building and maintenance (repairs) | Blasting abrasive | Sand |
| Rubbers and plastics | Raw Material Handling | Feeding hoppers (tripoli, diatomaceous earth) |
| Paints | Raw Material Handling | Feeding funnels (tripoli, diatomaceous earth, silica The Flour) |
| Soaps and cosmetics | Abrasive soaps, scouring powders | Silica flour |
| Asphalt and tarred cardboard | Application as filler and granules | Sand and aggregate, diatomaceous earth. |
| Chemicals for agriculture | Crushing and handling of raw materials. | Ores and phosphate rocks |
| Jewelry Store | Cutting, grinding, polishing, polishing | Semi-precious gemstones or abrasive stones |
| Dental Material | Abrasive sand, polishing | Sand abrasives |
| Car Repairs | Blasting abrasive | Sand |
| Boiler Scaling | Coal-Burning Boiler | Gray and concretions. |
Source: IARC 1997
Synonyms, Trade Names, and Formula
Source: IARC 1997
Synonyms, trade names and formula
Chemical Abstrat Service (CAS) Registration Number: 7631-86-9
Chemical Name: Silicon Dioxide
Molecular formula: SiO2.
Synonyms: Crystalline: cohesive, cristobalite, jasper, microcrystalline silica, quartz, quartizite, among others. Amorphous: Colloidal silica, diatomaceous earth, diatomite, fused silica fused silica, opal, silica gel, vitreous silica, among others
Trade names: Crystalline: BRGM, D&D, DQ12, Min-U-Sil, Sil-Co-Snowit. Amorphous: Aerosil, Celite, Ludox, Silcron G-910.
Origin and classification
Origin: Mineral, Biogenic or Synthetic
Classification:
a– crystalline forms
natural -, ß quartz; , ß1, ß2, tridymite; ß cristobalite; coesite;
stishovite; Moganite, Keatite.
synthetic – keatite; silica W; porosils
b– amorphous forms
natural – opal; biogenic silica; diatom lands; silica fibers;
vitreous silica.
synthetic – fused silica; pyrogenic or evaporated silica; precipitated silica;
colloidal silica; silica gel.
c- Rocks containing silica (> 90% SiO2)
quartzite, sandstone quartz, diatomite, porcelanite, corneal flint, geyserite
(Fondel, 1962; Coyle, 1982; Flörke & Martin, 1993)
-Quartz is the thermodynamically stable form of crystalline silica under ambient conditions. The vast majority of natural crystalline silica exists as a-quartz. The other forms exist in a metastable state. The nomenclature used is “a” for a low temperature phase and “ß” for a high temperature phase. The stability of silica polymorphs is related to temperature and pressure. Polymorph is a term used to describe materials with different crystalline atomic arrangements but of the same chemical composition. The quartz is the most stable in the temperatures and pressures that characterize the earth’s crust. Tridymite and cristobalite are formed under high temperatures, while coesite and stishovite are formed under high pressures.
Structure and chemical bonds
The basic structural unit of most forms of silica and silicate is a tetrahedral arrangement of 4 oxygen atoms around a centralized silicon atom, tetrahedral silicon, SiO4. Small variations in the orientation of the tetrahedral silicon cell with their respective one result in the development of new symmetry, producing the different polymorphs of silica, quartz, tridymite, cristobalite, coesite and stishovite. A totally random orientation of these units results in the amorphous varieties of the material.
This tetrahedral arrangement enables the formation of an infinite three-dimensional crystal lattice by sharing all oxygen atoms in a tetrahedron with neighboring groups. When some of the tetrahedron’s vertices do not bond, that is, oxygen atoms are free, a wide range of structural possibilities open, some of which are found in silicates. In structures for which all tetrahedron vertices are not shared, each unshared oxygen atom contributes a negative charge to the then formed anionic group, the equilibrium of these charges is the presence of cations (positive charges) in the structure of the silicate. In the figure is a schematic representation of the possible basic structures of silicates. Figure 2 shows the representation of the crystalline structure of quartz, cristobalite and tridymite.
Silica at ordinary temperatures is chemically resistant to many of the common reagents. In addition, it can withstand a wide variety of transformations under severe conditions such as high temperatures. The reactivity of silica strongly depends on its shape, pretreatment and subdivision status of the specific sample under study.
Recently generated crystalline free silica dust, such as sand blasting, rock drilling, tunneling and grinding operations, has higher toxicity to lung cells compared to older dust. Some studies that demonstrate this, describe that this increase in toxicity is due to the generation and presence of free radicals on the surface of the dust particle. Free oxygen radicals such as superoxide anion (O2.-) and hydroxyl radicals (.OH) are formed which are highly reactive in the presence of bivalent iron cation (Fe2 +) and traces of other metals.
The solubility, cleavage characteristics, morphology and surface properties of silica can influence its biological activity in organisms.
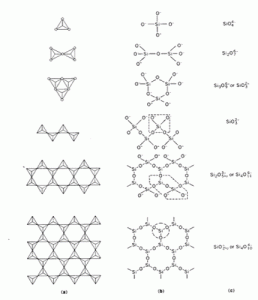
Figure 1. Schematic representation of basic silicate structures.
(a) SiO4 tetrahedron bonding forms;
(b) corresponding chemical binding standards;
(c) molecular formula. Si atoms appear attached only to 3 O atoms, the fourth Si-attached O atom is below the diagram plane.
Source: Kirk Otmer
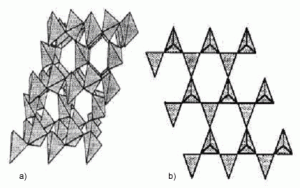
Figure 2. Representations of a-quartz polyhedral structures
(a) and tridymite and cristobalite, which is common to both (b).
Occupational Exposure
Occupational exposure occurs through inhalation by the worker of dust containing crystallized free silica.
Dust is any solid particle of any size, nature or origin, formed by grinding or other mechanical disruption of a solid original material, suspended or capable of being suspended in air. These particles usually have irregular shapes and are larger than 0.5 μm.
The location of particle deposition in the human respiratory system, Figure 1, depends directly on particle size:
- Inhalable particles smaller than 100 μm are able to penetrate through the nose and mouth.
- thoracic particles smaller than 25 μm are able to penetrate beyond the larynx;
- breathable particles smaller than 10 μm are able to penetrate the alveolar region.
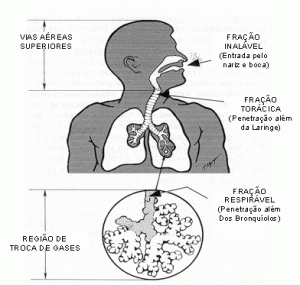
Dust can be classified in several ways. However, particle size classification is of fundamental importance when dealing with silica-containing dust, because the risk assessment for developing silicosis depends on the amount of inhaled crystallized free silica deposited in the respiratory bronchioles and pulmonary alveoli. The determining factors are: atmospheric concentration of respirable dust fraction and its free crystalline silica content, duration of worker exposure and individual susceptibility.
Toxic effects
Toxic effects on the human organism due to exposure to crystalline free silica dust depend on a number of variables:
- exposure type: breathable fraction composition, environmental dust concentration, crystalline free silica concentration, other breathable fraction minerals, particle size and exposure time;
- type of organic response: integrity of mucociliary system and immune responses; concomitance of other respiratory diseases; bronchial hyperreactivity.
The pathway of dust particles within the respiratory system is made up of the nose, mouth, pharynx, larynx, tracheobronchial tree and pulmonary alveoli, and settles in different regions depending on their aerodynamic diameter. In normal situations, the respiratory tract intercepts most inhaled particles by activating the defense and restoration mechanisms. However, this self-protection and damage repair capability has a limit. During occupational exposure, excessive dust deposition caused by frequent and continuous inhalation of this agent causes several adverse effects within the respiratory tract. In the tracheobronchial region the presence of dust stimulates an increase in mucus production to assist the work of conducting the cilia therein in the removal of particles. Prolonged stimulation of cells and mucus secretion glands may induce hypertrophy of these structures.
Particles that penetrate beyond the terminal bronchiol are rapidly ingested by cells called macrophages, whose function is to destroy foreign material. Some of the macrophages, with their ingested particles, are transported over the mucociliary lamina. Other macrophages die, releasing particles, active substances, and cellular debris that are ingested by new macrophages, and this process is repeated indefinitely. Macrophage life under normal circumstances is measured in terms of weeks or perhaps a month or more. Its life is shortened if the ingested particle is especially toxic, such as crystalline free silica, which due to its surface properties, kills the macrophage in a period of hours or days. Dust particles that lodge in the alveoli stimulate macrophage recruitment and accumulation in this area causing pulmonary tissue reactions. Studies have shown an increase in inflammation indicators mainly in the lungs of silicotic people. Collagen formation accompanies prolonged or chronic inflammation in most organs of the body. It is a part of the familiar scar tissue formation that can act on both the skin and the lung. Pulmonary fibrosis is a common sequela of chronic pulmonary inflammation. In addition, lung cells that are in contact with air have a high replacement or renewal rate, where cells with a partially damaged surface are rapidly replaced with new ones. Due to the rapid regeneration of lung cells, there is probably greater vulnerability to carcinogenic changes due to the presence of dust. Based on all of the above considerations, it can be anticipated that dust deposited in the lungs may induce:
- little or no reaction;
- mucus overproduction and mucus secretion glands hypertrophy,
- macrophage recruitment;
- chronic proliferation or inflammatory reaction;
- fibrosis;
- cancer.
Cancer
Inhaled crystalline free silica in the form of quartz or cristobalite from occupational exposures is carcinogenic to humans according to the World Health Organization (IARC).
An IARC Working Group analyzed a series of epidemiological studies conducted in various fields of activity such as mining, extraction and work with granite, ceramics, pottery, refractory, industrial processes with diatomaceous earth, smelting and others.
In epidemiological studies relating silicosis and lung cancer risk, it was found that a silicotic is 1.5 to 6 times more likely to acquire lung cancer than a non-silicotic.
In most studies, the increased risk gradient was observed in relation to dose, cumulative exposure, duration of exposure or the presence of radiologically defined silicosis.
The Working Group concluded that the evidence found in these studies was sufficient to substantiate the increase in lung cancer from inhalation of crystalline free silica resulting from occupational exposure.
The mechanisms that induce cancer formation caused by crystallized free silica are still being studied. There is a greater body of evidence demonstrating that the persistent process of lung inflammation generates oxidizing substances that result in genotoxic effects on the lung parenchyma.
Bibliography
- Algranti E, By Capitani E M, Bagatin E. Pathology of Labor According to Respiratory Tract. In: Work Pathology. 2nd ed. Sao Paulo-SP. Atheneu Publisher. 1995.p.87-137.
- Algranti E. Occupational lung disease in Brazil. In: Daniel E. Banks and John E. Parker, editors. Occupational Lung Disease. London, GB. Chapman & Hall 1998. p. 105-115.
- Guthrie GD. Mineralogical factors affect the biological activity of crystalline silica. Appl.Occup. Environ. Hyg. 1995; 10 (12): 1126-1131.
- Hearl FJ. Identification monitoring and control of exposures. In: Daniel E. Banks and John E. Parker, editors. Occupational Lung Disease. country town Chapman & Hall 1998. p. 36-525.
- IARC (International Agency for Tesearch on Cancer). Silica Some Silicates Coal Dust and Para-Aramid Fibrils. IARC Monographs on the Evaluation of the Carcinogenic Risk of Chemicals to Humans. Vol.38, Lyon, France. 1997
- Kirk Otmer. Encyclopedia of Chemical Technology. Silica. Volume 21:
- p 977-1005 Fourth Edition. The Willy Interscience Publication. John Wiley & Sons. Inc. 1997.
- Lippmann Morton. Exposure Assessment Strategies for Crystalline Silica Health Effects. Appl.Occup. Environ. Hyg. 1995; 10 (12): 981-990.
- Patty’s. The pulmonary effects of inhaled inorganic dust in: Patty’s Industrial Hygiene and Toxicology, Vol. 1, Chap. 7, 1978.
- Santos AMA, Bon AMT, Amaral NC. Environmental evaluation of crystallized free silica carried out in the sand classification laboratory of the São Paulo State Institute of Technological Research S / A -IPT. Technical Report RT / 02, Fundacentro, São Paulo, 1998.
- Vallyathan V, Shi X. The role of oxygen free radicals inoccupational and environmental lung diseases. Environmental Health Perspectives. Vol.105, Supplement 1 February 1997
| HAZARD PREVENTION AND CONTROL IN THE WORK ENVIRONMENT AIRBORNE DUST This document is intended for training in preventing the generation of mineral dust and controlling its spread in the workplace, in a didactic manner, with updated concepts. |
| Exposure to Silica and Silicosis This text describes epidemiological data, presentation forms, complications and the most commonly used tools for the diagnosis of the disease. Some relevant bibliographical references are suggested for further reading. |
| Basic Measures for the Prevention of Dust Exposure containing Crystallized Free Silica The text deals with basic measures for prevention and control of occupational exposure to silica involving source control, enclosure and ventilation, working practices and personal measures. |
-
Phone
+55 (11) 3388-3534
-
Rua Muniz de Souza, 306
Aclimação, São Paulo - SP
-
E-mail:
zirtec@zirtec.com.br
